A new study from IMA researchers Matt Halma and Dr. Joseph Varon introduces DARE-SAFE, a first-of-its-kind system for comparing vaccine and drug safety—showing COVID-19 vaccines had 63x higher reported death rates than flu shots.

No matter where you stand on vaccines, one thing is hard to ignore: there’s a serious safety data problem. For decades, pharmacovigilance systems like the Vaccine Adverse Event Reporting System (VAERS) and the FDA Adverse Event Reporting System (FAERS) have struggled to provide reliable comparisons between drugs or detect meaningful safety signals. Organizations like Children’s Health Defense have documented these failures for years.
The rollout of the COVID-19 mRNA vaccines pushed the issue into the spotlight. Suddenly, even those who had never questioned vaccine safety began asking why adverse events were being ignored, denied, or dismissed.
Now, IMA researchers Matt Halma and Dr. Joseph Varon have teamed up to create a tool that confronts this problem directly. The DARE-SAFE method—short for Denominator-Adjusted Rate Estimates of Substance Adverse Events Frequency Evaluation—is the first comparison of adverse event reporting rates across different vaccines and pharmaceuticals.
DARE-SAFE: Denominator-Adjusted Rate Estimates of Substance Adverse Events Frequency Evaluation in Pharmaceuticals and Vaccines – Matt Halma, Joseph Varon
What the DARE-SAFE Method Measures
The goal of DARE-SAFE is simple: to standardize how we evaluate adverse event reports. The method tackles the biggest weakness of passive surveillance systems: the lack of denominator data. It achieves this by incorporating dose and prescription numbers into its calculations.
Researchers used:
- OpenVAERS and CDC dose data for vaccines (2006–2022)
- FAERS reports and ClinCalc prescription data for the top 250 drugs (2022)
By analyzing both vaccines and the top 250 most commonly prescribed drugs, the method establishes baselines for what “normal” reporting patterns look like. Now, for the first time, you can compare a vaccine’s reporting rate against these baselines to see if it’s within expected ranges or signaling something unusual.
The result: DARE-SAFE is a standardized, dynamic system that quantifies reports per dose and identifies when products deviate from established patterns.
Key Findings From the Study
This study delivers a powerful one-two punch for science: it not only introduces the DARE-SAFE system to fix a long-ignored flaw in pharmacovigilance but also uses it to reveal striking disparities in vaccine and drug safety reporting.
Vaccines:
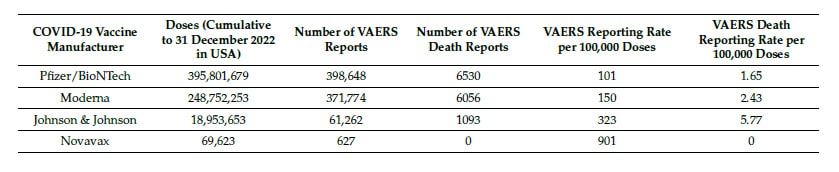
- COVID-19 vaccines showed 63x more VAERS death reports per dose than flu vaccines.
- They also exhibited nearly 19x more total adverse event reports per dose.
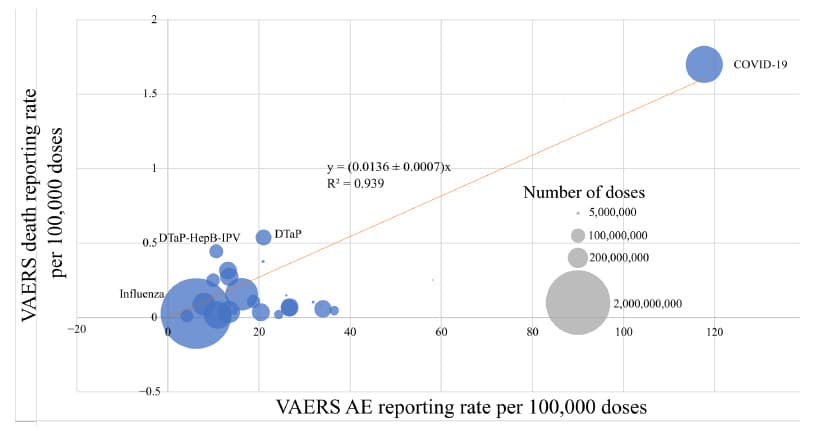
Pharmaceuticals:
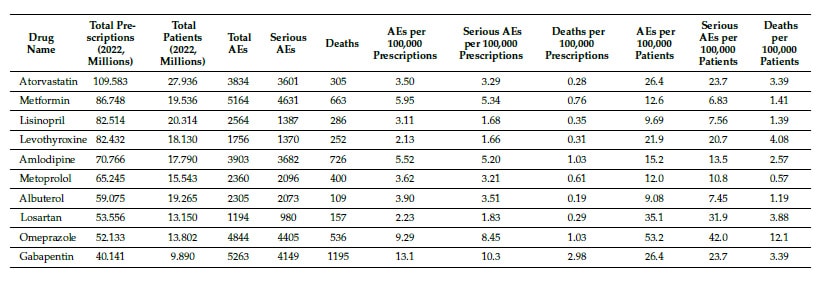
While the COVID vaccine results are the most dramatic, the pharmaceutical findings also play an important role. By applying the same DARE-SAFE method to the top 250 most prescribed drugs in the U.S., the researchers were able to establish a consistent pattern of reporting: roughly 26 adverse event reports for every death report.
That consistency helps set a benchmark for what “normal” looks like in post-market surveillance—something that regulators have overlooked for decades. Table 3 below shows AE rates and deaths for the ten most commonly prescribed prescription drugs in the USA.
In contrast, the COVID-19 vaccine reporting ratios weren’t just higher; they were in a different universe. Without the pharmaceutical data, that distinction would be harder to see. And, as Table 2 shows below, there are even major differences in outcomes even when comparing different COVID injections:
Why This Matters
The current post-market safety landscape relies heavily on incomplete data and inconsistent reporting. With DARE-SAFE, regulators and researchers finally have a way to standardize safety comparisons using real-world surveillance data.
This method gives healthcare professionals and the public:
- A clear benchmark for comparing products
- A tool for detecting anomalies in safety signals
- A framework to evaluate post-market safety without requiring full clinical trials
And unlike incidence estimates, these reporting rates reflect what’s being flagged by the public and clinicians—not what’s being estimated by models.
What Happens Next
The study authors are calling for further refinement of DARE-SAFE and broader application across pharmacovigilance efforts. This includes:
- Adapting the model for new vaccines and drug approvals
- Applying the framework to international surveillance systems
- Integrating the method into ongoing regulatory oversight
The Independent Medical Alliance plans to continue supporting data transparency and safety science through ongoing analysis and collaboration.
Too many studies stay buried in academic journals. We believe research like this deserves a public spotlight—especially when it offers solutions to long-standing safety concerns. The data is here. The patterns are clear. And now, with a standardized method, there’s no excuse not to act.
This is the kind of scientific work that empowers patients, challenges regulatory complacency, and restores integrity to public health decision-making. It’s also the kind of donor-supported research that drives our mission forward. With each peer-reviewed study, we move one step closer to a world where practitioners and patients have the data they need to make truly informed decisions.
Explore more of our original research and breakthrough studies below:
- Nature’s Overlooked Osteoporosis Solution: New Study Finds Up to 6% Bone Density Gains
- Feeling Anxious? New Study Shows Ashwagandha, CBD, and Saffron Effective for Anxiety Disorders
- Tainted Vials: New Study Reveals Massive DNA Contamination in COVID Shots
- New Peer-Reviewed Study Asks: Were COVID Injections Engineered to Harm?
- Hybrid Harms: When COVID Shots and Infections Compound





